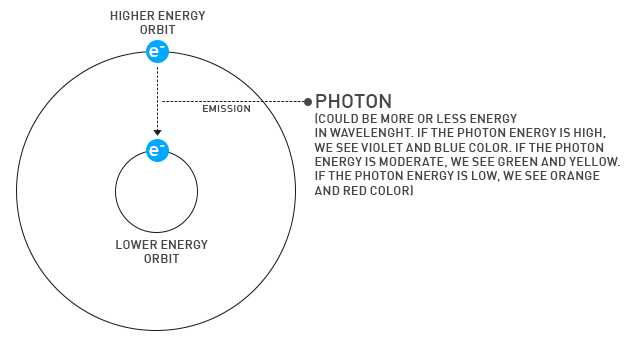
There are many electrons in each atom or molecule, and these electrons can be found at various energy levels. In order for an electron to move from a lower energy level to a higher energy level, it needs to receive energy from the outside. But an electron can go from a higher energy level to a more stable lower energy level required by the law of "conservation of energy" and emit photons (light packs) around it. The frequency of these photons creates the emission spectrum. Some of them are visible in the visible range (400-700 nanometer wavelength) so, we can see them with bare eyes.

Emission in Octane means using any object as a light source (Temperature with Kelvin). In doing so, it simply formulates a natural phenomenon called "Blackbody Radiation" under the name "Blackbody Emission". Thus, any object in your scene becomes a light source.